Description
In human prostate cancer, the ERG oncogene is frequently overexpressed due to chromosomal translocations involving ERG and regulatory sequences of the TMPRSS2 or other androgen responsive genes. In particular, the TMPRSS2:ERG fusion gene has been found to be the most frequent gene rearrangement in prostate cancers, occurring in 45-65% of North American patients.
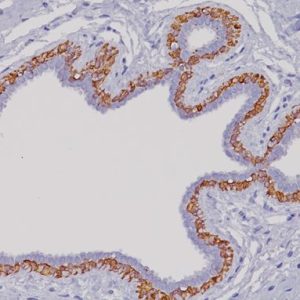
A mouse monoclonal anti-ERG antibody was developed with an unprecedented 99.9% specificity for detecting prostatic adenocarcinoma. The report shows strong correlation between the expression of the ERG protein and the presence of TMPRSS2:ERG rearrangement and a remarkable concordance (96.5%) of ERG positive prostatic intraepithelial neoplasia (PIN) and ERG positive carcinoma in prostatectomy specimens.
Therefore, as a hallmark of the TMPRSS2:ERG chromosomal translocation, ERG expression offers a rare, but definitive marker of adenocarcinoma of prostatic origin, and unique opportunities to indicate oncogenic activations in PIN, to stratify prostate cancer patients for ERG oncogene status and to monitor treatment efficacy. Given the ease of performing IHC vs. FISH, ERG protein expression in formalin-fixed paraffinembedded (FFPE) tissues may be an extremely useful tool for the routine identification of the ERG gene rearrangement and diagnosis of prostatic adenocarcinoma.
Further utility for the mouse monoclonal anti-ERG antibody has been demonstrated recently in detecting endothelial malignancies, such as Kaposi sarcoma. ERG (M), 2X may be combined with AMACR (RM), 2X to form a primary antibody combination (see technical notes).
Note: ERG [9FY] was developed by the Center for Prostate Disease Research in association with the Henry M. Jackson Foundation, Rockville, Maryland. Patent Pending.
SPECIFICATIONS
Specifications
| WEIGHT | N/A |
|---|---|
| DIMENSIONS | N/A |
| INTENDED USE | IVD |
| SPECIES REACTIVITY | Human |
| SOURCE | Mouse Monoclonal |
| CLONE | 9FY |
| ISOTYPE | IgG1 |
| ANTIGEN | ERG |
| LOCALIZATION | Nuclear |
| POSITIVE CONTROL | ERG positive prostate cancer and/or PIN glands. |
DATASHEETS & SDS
REFERENCES
1. Petrovics G, Liu A, Shaheduzzaman S, Furasato B, Sun C, Chen Y, Nau M, Ravindranath L, Chen Y-D, Dobi A, Srikantan V, Sesterhenn IA, McLeod DG, Vahey M, Moul WJ, Srivastava S. Frequent over-expression of ETS related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 24, 3847-3852 (2005).
2. Kumar-Sinha C, Tomlins SA, Chinnaiyan AM. Recurrent gene fusions in prostate cancer. Nat Rev Cancer 8, 497-511 (2008).
3. Furusato B, Tan SH, Young D, Dobi A, Sun C, Mohamed AA, Thangapazham R, Chen Y, McMaster G, Sreenath T, Petrovics G, McLeod DG, Srivastava S, Sesterhenn IA. ERG oncoprotein expression in prostate cancer: clonal progression of ERG positive tumor cells and potential for ERG based stratification. Prostate Cancer and Prostatic Diseases 13, 228-237 (2010).
4. Mohamed AA, Tan S-H, Mikhalkevich N, Ponniah S, Vasioukhin V, Bieberich CJ, Sesterhenn IA, Dobi A, Srivastava S, Sreenath LT. Ets Family Protein, Erg Expression in Developing and Adult Mouse Tissues by a Highly Specific Monoclonal Antibody. Journal of Cancer 1, 197-208 (2010).
5. Miettinen M, Wang Z-F, Paetau A, Tan S-H, Dobi A, Srivastava S, Sesterhenn IA.: ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. American Journal of Surgical Pathology 35, 432-441 (2011).
6. Mohamed AA, Tan S-H, Sun C, Shaheduzzaman S, Hu Y, Petrovics G, Chen Y, Sesterhenn IA, Li H, Sreenath T, McLeod DG, Dobi A, Srivastava S. ERG oncogene modulates prostaglandin signaling in prostate cancer cells, Cancer Biology and Therapy 11, 410-417 (2011).
7. Hameed O, Humphrey PA. Immunohistochemistry in diagnostic surgical pathology of the prostate. Semin Diagn Pathol 22, 88-104 (2005).
8. Trpkov K, Bartczak-McKay J, Yilmaz A. Usefulness of cytokeratin 5/6 and AMACR applied as double sequential immunostains for diagnostic assessment of problematic prostate specimens. Am J Clin Pathol 132, 211-220 (2009).
9. Center for Disease Control Manual. Guide: Safety Management, NO. CDC-22, Atlanta, GA. April 30, 1976 “Decontamination of Laboratory Sink Drains to Remove Azide Salts.”
10. Clinical and Laboratory Standards Institute (CLSI). Protection of Laboratory workers from occupationally Acquired Infections; Approved guideline-Third Edition CLSI document M29-A3 Wayne, PA 2005.






Reviews
There are no reviews yet.